Use of Booklet Labels on Investigational Medicinal Products (IMPs) | ISPE | International Society for Pharmaceutical Engineering

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library
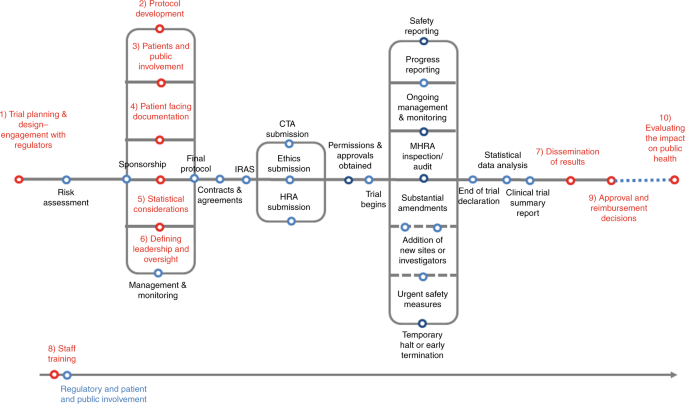
Effective delivery of Complex Innovative Design (CID) cancer trials—A consensus statement | British Journal of Cancer