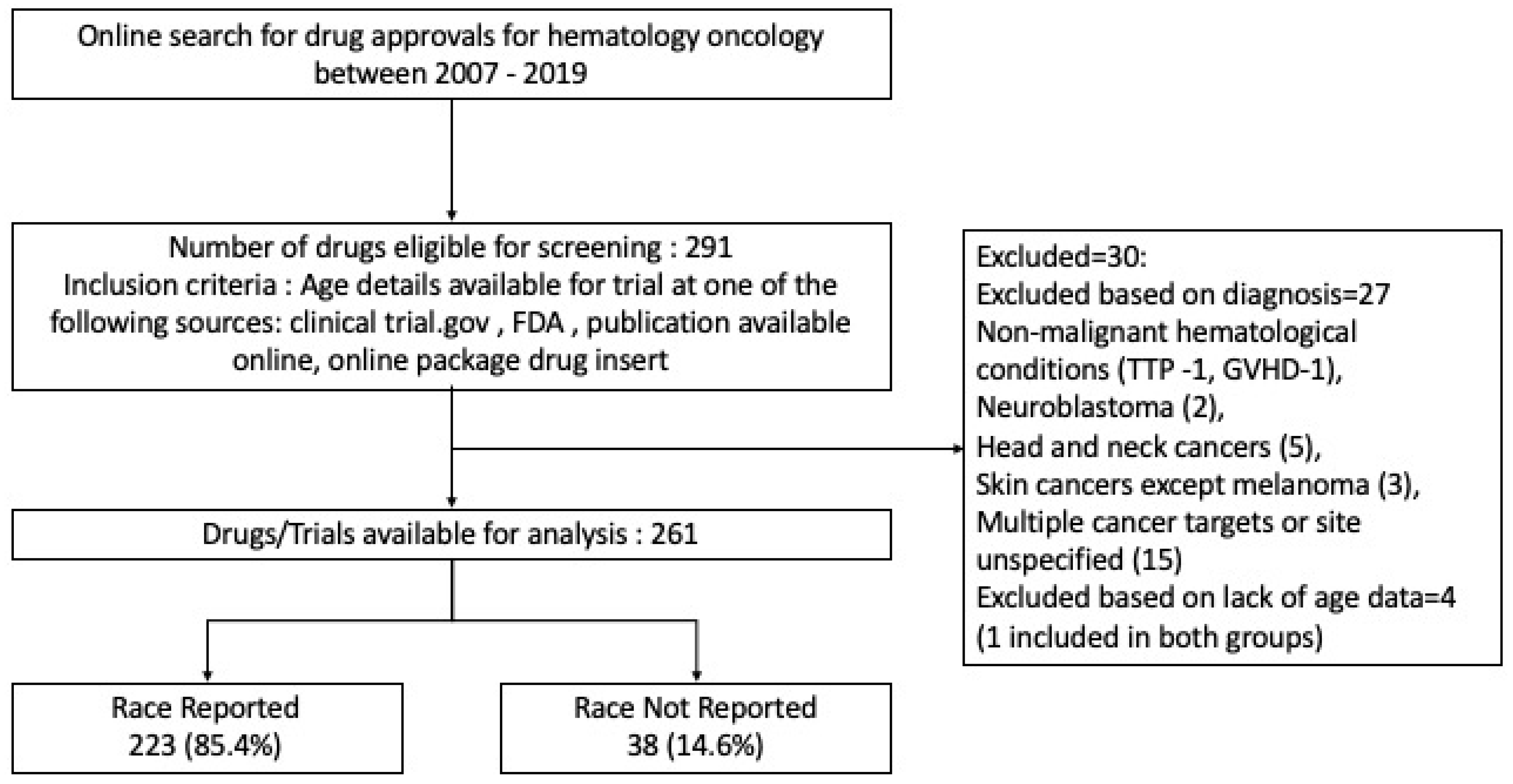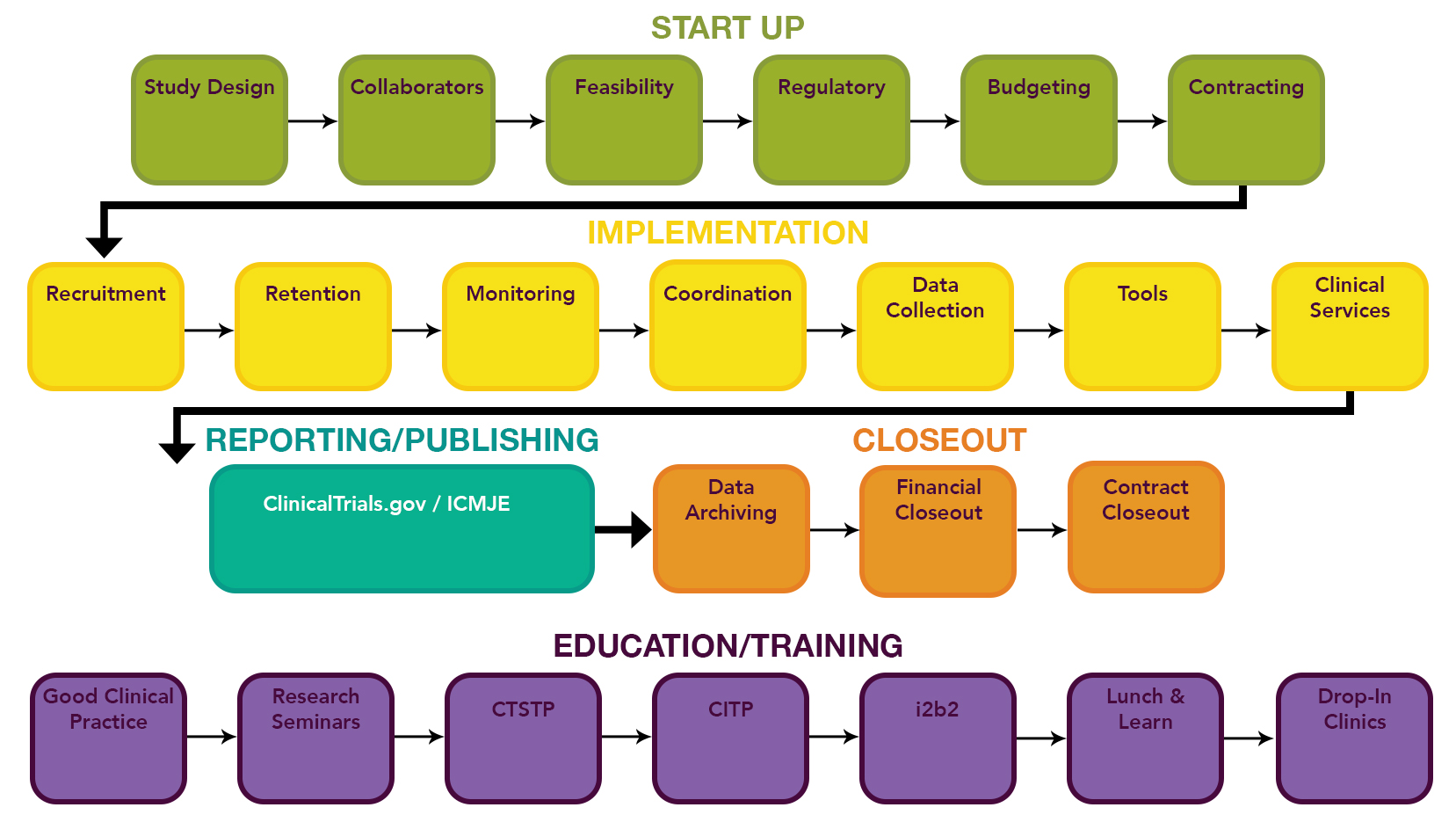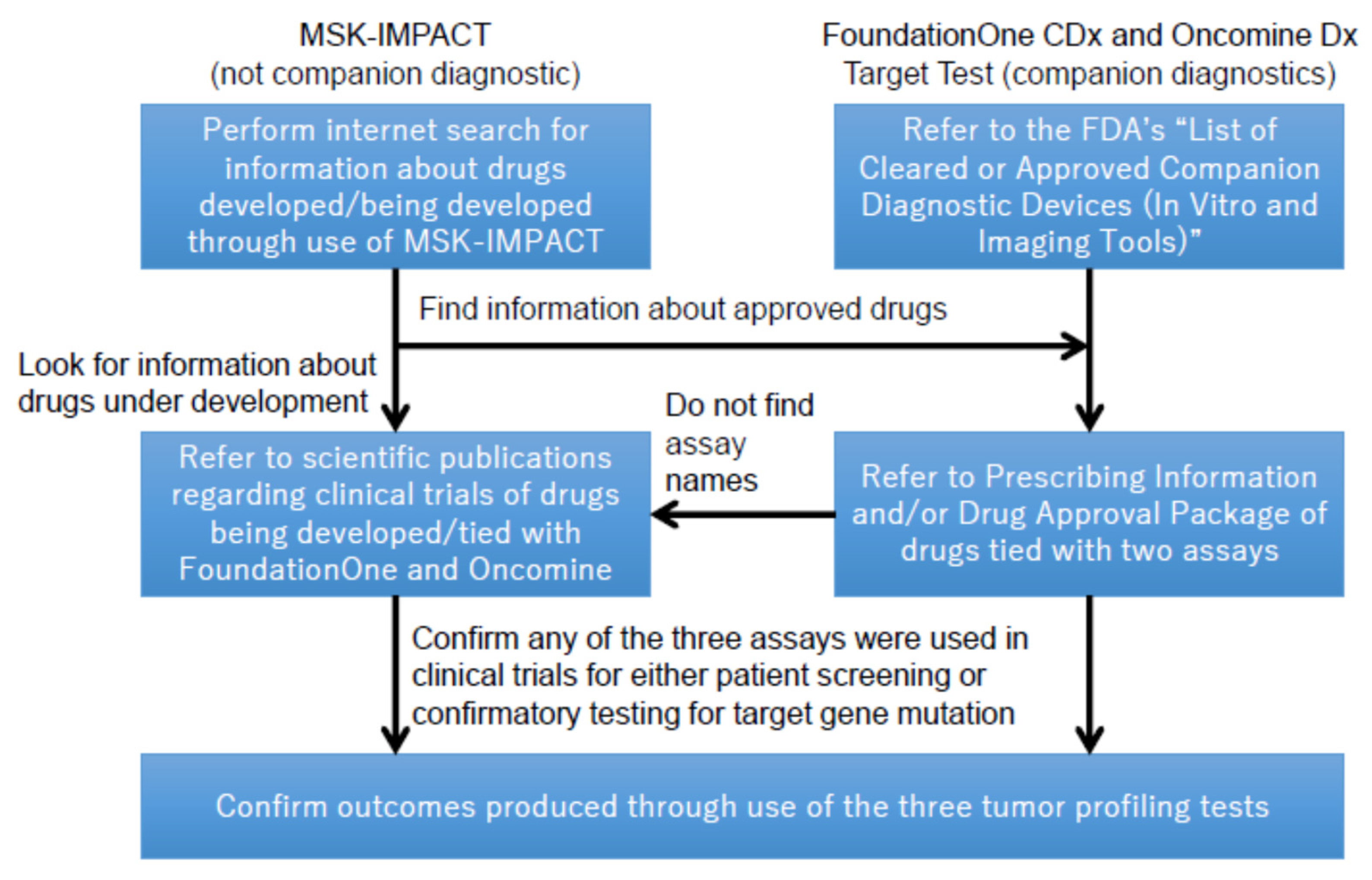
Cancers | Free Full-Text | Regulations, Open Data and Healthcare Innovation: A Case of MSK-IMPACT and Its Implications for Better Cancer Care | HTML

Center Watch Clinical Trials Listing; http://www.centerwatch.com/ - 1999 - Diabetes/Metabolism Research and Reviews - Wiley Online Library

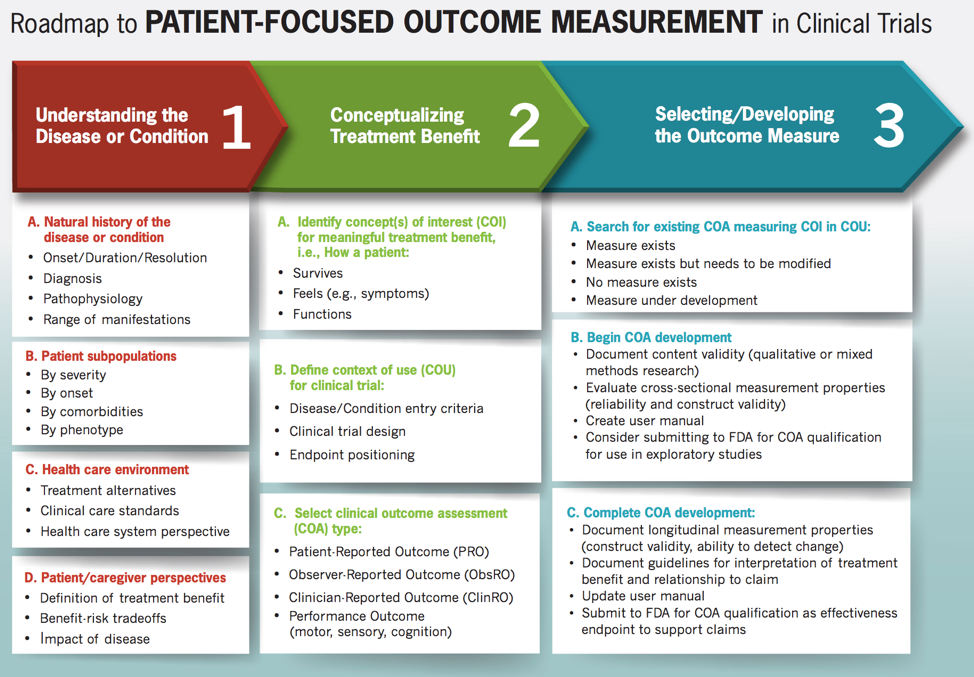
FDA roadmap to patient-focused outcome measurement in clinical trials. 3 | Download Scientific Diagram

Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs | Journal of the American College of Cardiology

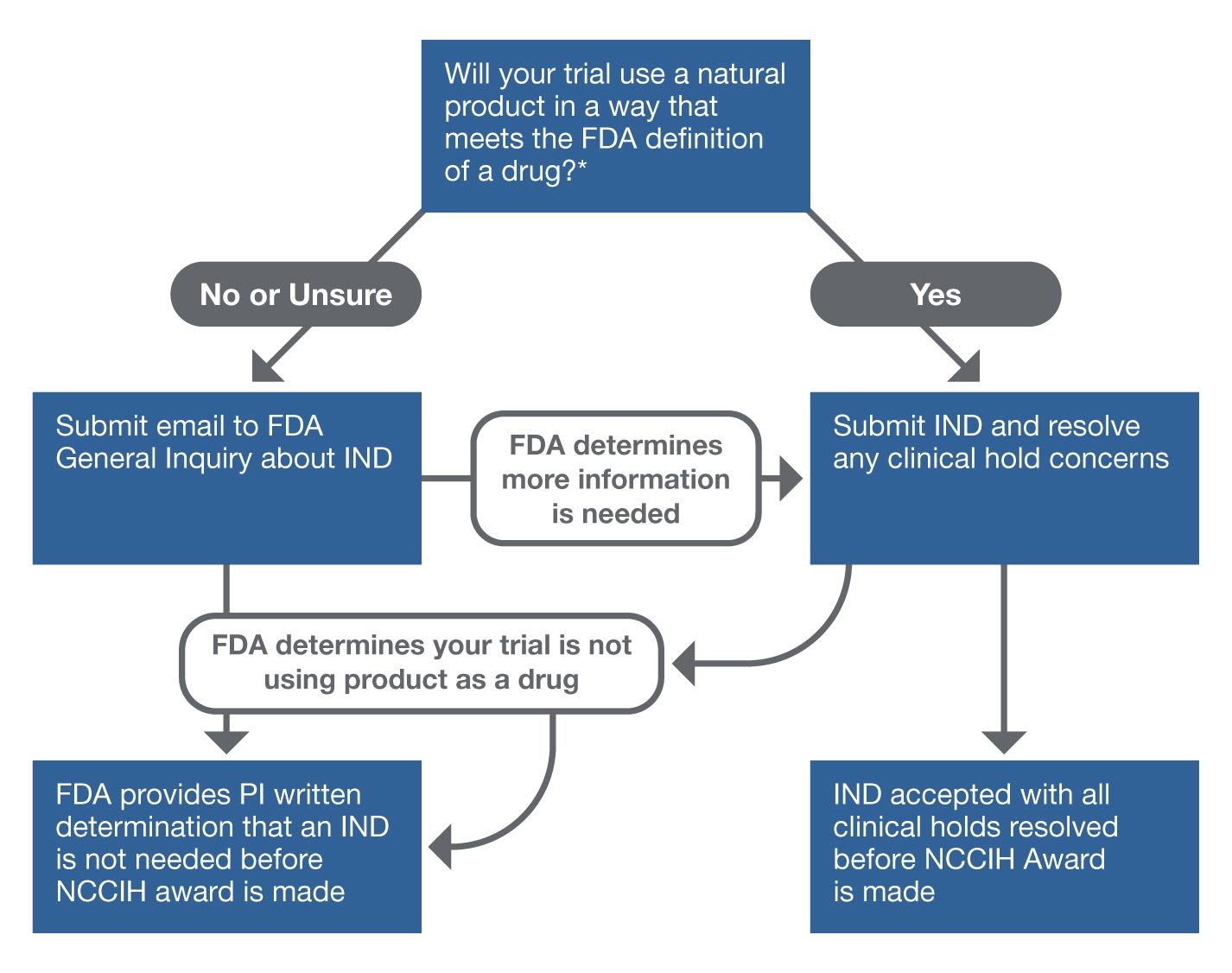
Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO