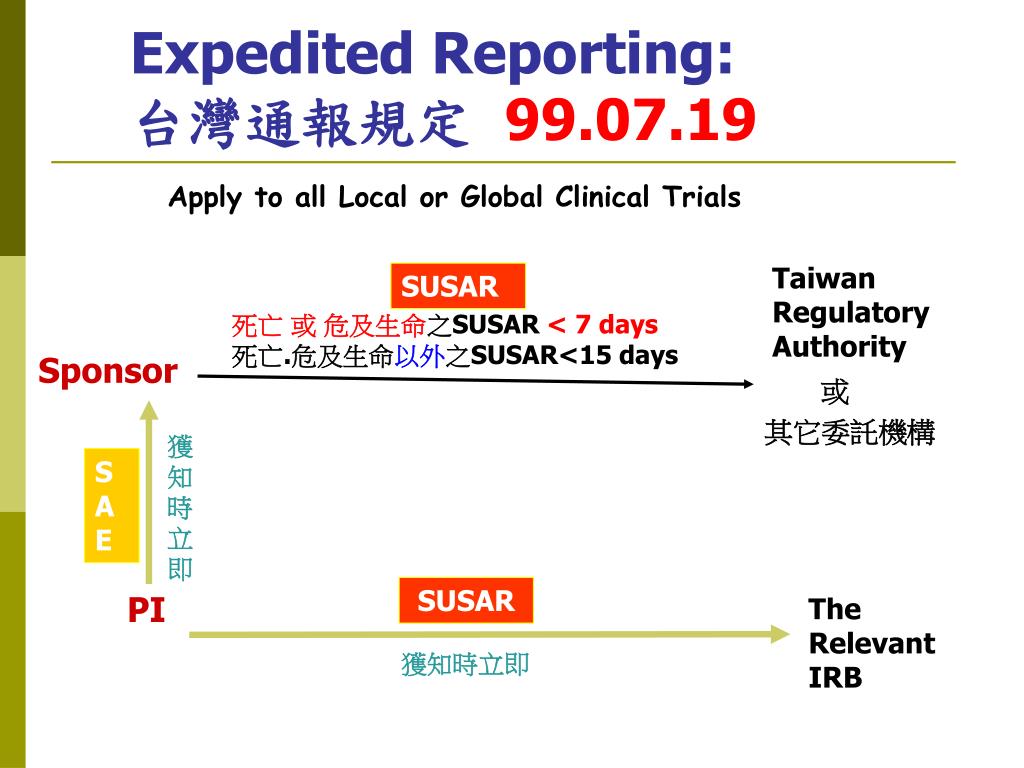
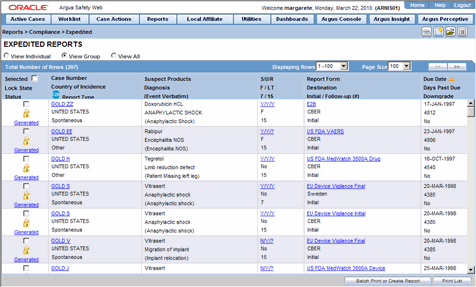
Expedited Reporting-Personnel Communication Flow Chart (Amended: 13 Nov... | Download Scientific Diagram

Book 6: 2021 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC

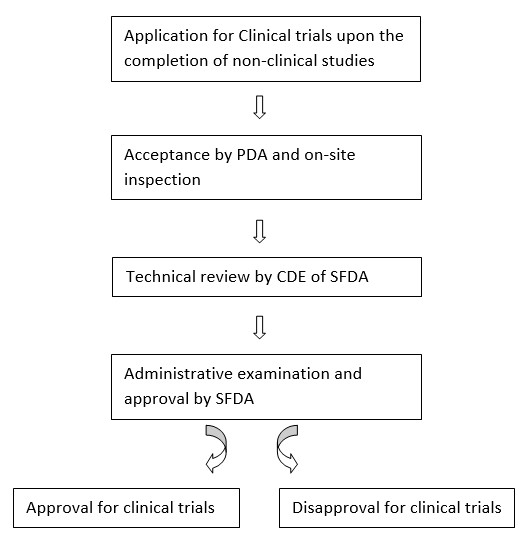
THE NEW STANDARDS AND PROCEDURES FOR THE EXPEDITED REPORTS OF SAFETY DATA DURING CLINICAL TRIALS RELEASED BY CHINA - International Drug Safety CROs

Pharmacovigilance for clinical trials in India: Current practice and areas for reform Brahmachari B, Fernandes M, Bhatt A - Perspect Clin Res
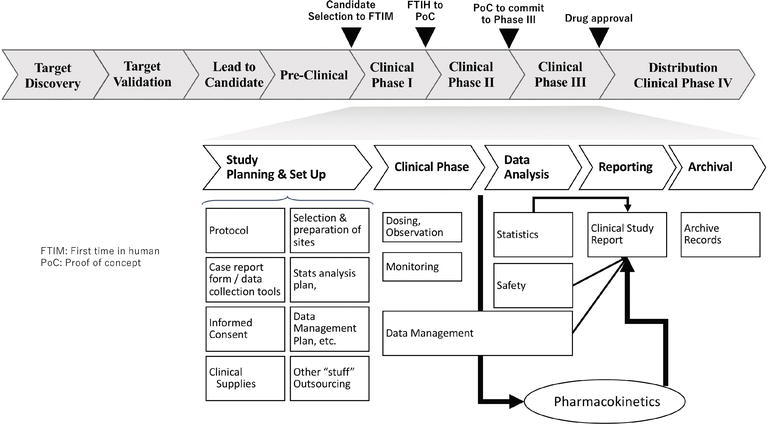
Frameworks for Evaluating Qualitative and Quantitative Information on Adverse Drug Events throughout Development through to Marketing | IntechOpen