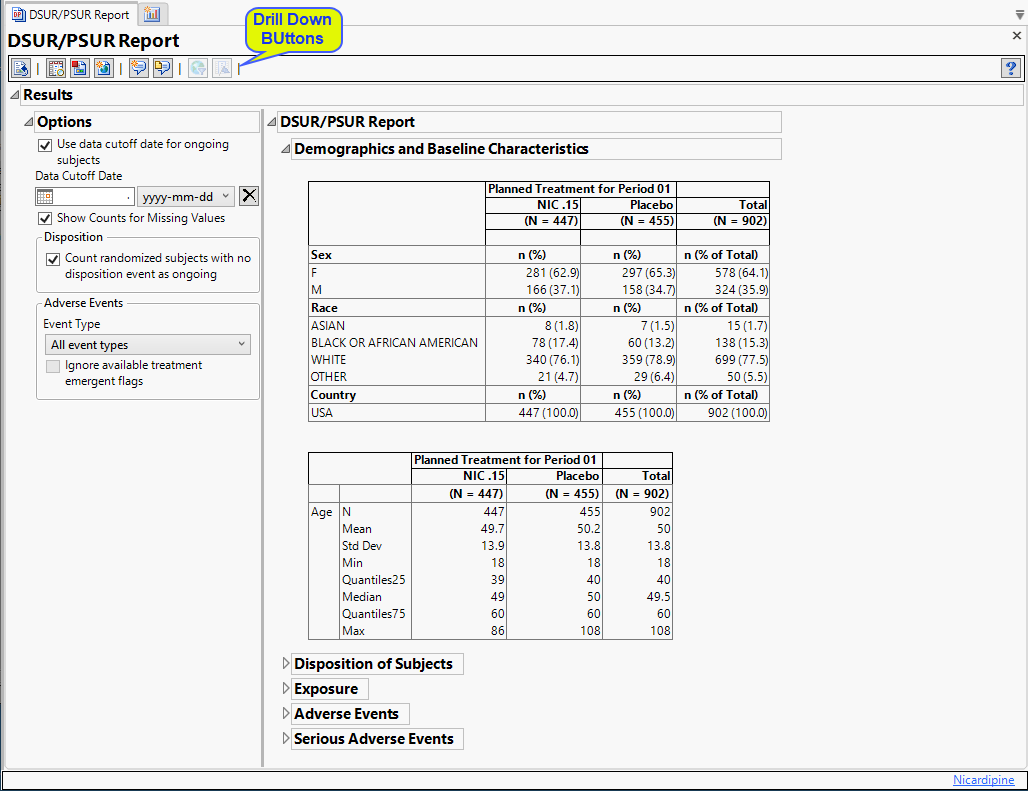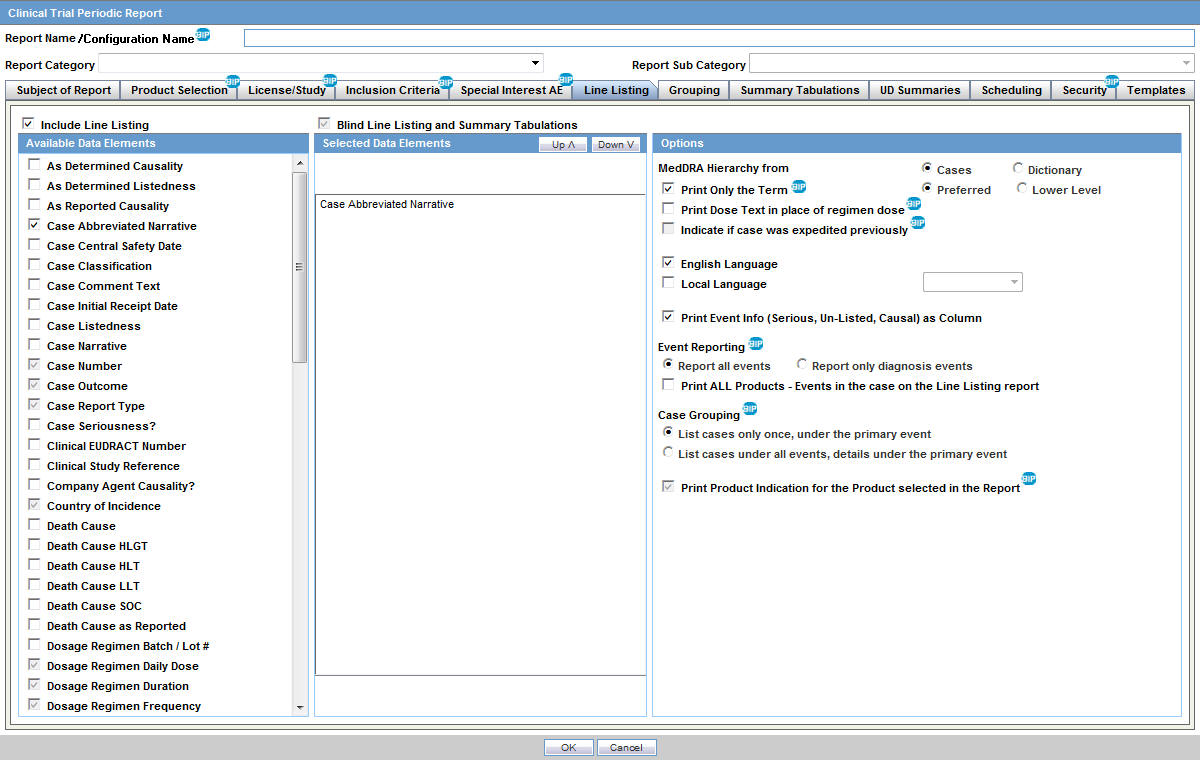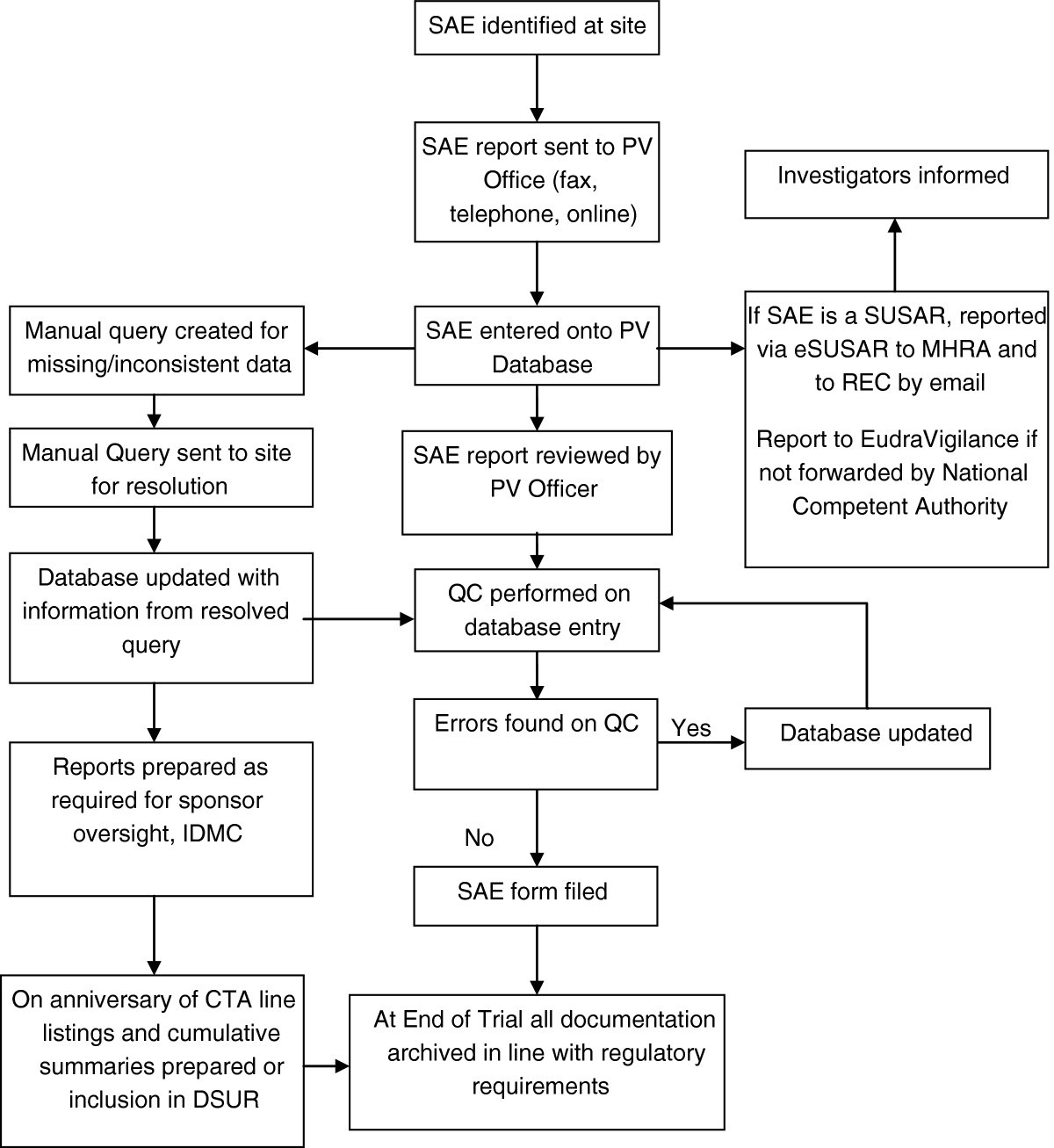
Implementing a centralised pharmacovigilance service in a non-commercial setting in the United Kingdom | Trials | Full Text

PDF) The Development Safety Update Report (DSUR): Harmonizing the Format and Content for Periodic Safety Reporting During Clinical Trials Report of CIOMS Working Group VII The Development Safety Update Report (DSUR): Harmonizing